http://www.collembola.org/publicat/integum/epicut.htm
Last updated on
2012.12.06
by Frans Janssens
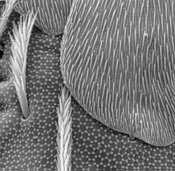
Fig.1. Hexagonal epicuticular surface structure in Tomocerus sp.
Ghiradella, H.T. © 2000
 |
Frans Janssens,
Department of Biology, University of Antwerp, Antwerp, B-2020, Belgium
Jean-Auguste Barra,
Laboratoire de Zoologie, Université Louis Pasteur, Strasbourg, 67000, France
Luc De Bruyn,
Department of Biology, University of Antwerp, Antwerp, B-2020, Belgium
In construction.
Abstract
The formation of the surface ultrastructure of the laminate cuticula of
Collembola
and more in particular the formation of the regular (mostly hexagonal)
pattern on the surface of the epicuticula is
based on an epidermal by exocytosis regulated receptor mediated
deposition of lipocuticulin micellae.
Introduction
Fig.n. Epicuticular surface structure of dorsal metanotal bladder-like vesicle of male Sminthurides sp.
Palacios-Vargas, J. © 2000.
 |
The ultrastructure of the surface of the integument of the
Collembola is one of their most striking features (Hopkin, 1997:51).
While it is possible to make the epicuticular surface structure visible in
light microscopy (fig.1'),
the details of the surface texture was revealed in detail by scanning and transmission
electron microscope studies by Massoud in 1969 and by Paulus in 1971
(Ghiradella & Radigan, 1974:301;
Eisenbeis & Wichard, 1985:218).
The basic pattern is a regular hexagonal tesselation of microtubercles
interconnected by ridges (Fig.1, Fig.n, Fig.x, Fig.y)
(Ghiradella & Radigan, 1974:302;
Massoud & Barra, 1980:251;
Eisenbeis & Wichard, 1985:205(Tafel 92 fig.d));
Palacios-Vargas, 2000:103(Foto 3)).
The hexagonal pattern occurs on all integumental surfaces,
even on the surface of the ocelli (Ghiradella & Radigan, 1974:302).
Barra, 1971:322-353 described four variants of the
ocellar surface ultrastructure.
The hexagonal pattern does not occur
- on the dorsal face of the unguis of Folsomia candida,
- on the retractile vesicles of the ventral tubus of Pogonognathellus flavescens
(Eisenbeis & Wichard, 1985:207(Tafel 93 fig.d)),
- on the rami of the retinaculum of Pogonognathellus flavescens
(Eisenbeis & Wichard, 1985:207(Tafel 93 fig.d)),
- on the teeth of the mucro of Orchesella villosa
(Eisenbeis & Wichard, 1985:209(Tafel 94 fig.d)),
- on the vesicles of the post antennal organ of Onychiurus spec.
(Eisenbeis & Wichard, 1985:213(Tafel 96 fig.d)),
- on the pseudocelli of Onychiurus spec.
(Eisenbeis & Wichard, 1985:213(Tafel 96 fig.f)),
- on the post antennal organ of Isotoma aff. saltans
(Eisenbeis & Wichard, 1985:227(Tafel 103 fig.f & fig.g)),
- on the distal part of the labrum of Isotomurus palliceps
(Eisenbeis & Wichard, 1985:228(Tafel 104 fig.e)).
|
Fig.x. Epicuticular surface structure of Tomocerus sp.
Plant, N. © 2000
 |
In Pogonognathellus flavescens,
the centre to centre distance of the hexagons is about 750 nanometer
on the body and about 300 nm on the eye;
[on the body] the ridges are about 150 nm wide and 100 nm high
(Ghiradella & Radigan, 1974:302).
Not only hexagonal but square arrangements,
such as in Anurida maritima, also occur.
In species at risk from desiccation, the microtubercles are closely apposed
and the cuticle is thickened to lower its permeability and reduce transpiration
of water.
Adaptations of the arrangement of the microtubercles to cold do not seem
to occur.
Epicuticular surface structure of Megalothorax sp.
Walter, D.E. © 1999
 |
In this paper, it will be shown that,
while there are species-specific differences in the surface pattern,
the formation of the ultrastructure is merely controled by the
electro-chemical and physical characteristics of the film formation process
of the by the epidermal cells secreted epicuticular material.
This might indicate that the ultrastructure should
not be used for taxonomic purposes,
as already has been suggested (Hopkin, 1997:55).
Exceptions do exists: the epicuticular ultramorphology of the
species of the Coenaletidae (see Fig.5)
is considered as characteristic
to the family (Palacios-Vargas, 2000:103(Foto.2),104).
This family-specific ultrastructure might be an adaptation to the marine
littoral and commensal life with Coenobita clypeata
and might function as a means to form a distributed plastron on the body
of suddenly in water submerged animals.
To be clarified:
In Tetradontophora? Eisenbeis found an
ultramorphological structure similar to Coenaletidae (Eisenbeis:199Y:p).
Epicuticular surface structure observation in light microscopy
Fig.lm. Epicuticular surface structure of Tomocerus sp.
Janssens, F. © 2004
 |
First of all, especially when observing the cuticular surface structures in
light microscopy, the pattern of epicuticular microtubercles
should not be confused with the pattern of exocuticular tubercles.
The pattern of epicuticular microtubercles is superimposed upon the
pattern of exocuticular tubercles.
The epicuticular ultrastructure can be recognised using light microscopy
techniques.
However, the details of the ultrastructure cannot be made visible due to the
limited capabilities of the visible light to resolve the tiny substructures
(the wavelength of the light is equal or
larger than the size of the details of the ultrastructure).
Using a magnification of 1000 times (ocular lens 10x, object lens 100x)
(the conventional Abbe limit), a thin section of a specimen of
Tomocerus sp., embeded in paraplast, and treated with a
histological hemaluin-eosin colouring technique,
was observed applying an immersion oil phase-contrast illumination system.
A network of interconnected microtubercles in a hexagonal pattern is
clearly revealed (fig.1m).
Fig.y. Epicuticular surface structure of Entomobrya sp.
Plant, N. © 2000
 |
The nonchitinous epicuticula is composed of substances that
are also constituents of the exocuticula (Wigglesworth, 1933b).
According to
Kuhnelt (1928, 1928a), the surface film (Grenzlamelle) of the exocuticula
is highly resistant to acids, but when heated in caustics
it is saponified and can be shown
to contain fatty acids and cholesterin (Snodgrass, 1935).
The epicuticula differentiates into four layers
(Massoud & Barra, 1980:251):
a cement layer,
a wax layer,
an external or outer epicuticular layer (of polyphenolic resins)
and
an internal or inner epicuticular layer
(of cuticulin
= a mixture of tanned lipoproteins and an
enzyme phenoloxidase,
that produces extra tanning in case of
damage of the epicuticula - self-repairing).
Summary of the nomenclature used by several authors with respect to the
laminate structure of the epicuticula (Edney, 1977:48-50):
the inner epicuticula, 300 to 1000 nm thick in Rhodnius,
rich in lipids impregnating a highly tanned protein component.
It is
the "cuticulin layer" of Wigglesworth (1947),
the dense layer or protein epicuticle of Locke (1961),
the inner epicuticle of Weis-Fogh (1970);
the outer epicuticula, 17 nm thick,
highly resistant and lipid rich.
It is
the resistant layer of Wiggleworth (1947),
the paraffin layer of Dennell & Malek (1955),
the cuticulin layer of Locke (1961) and Filshie (1970),
the outer epicuticle of Weis-Fogh (1970);
the wax layer, 10 nm thick,
lipid, hard, and hydrophobic.
It is
the surface monolayer or oriented lipid layer of Locke (1966),
the outer epicuticle or superficial layer of Filshie (1970);
the cement layer, 30 to 100 nm thick,
a stabilised mucopolysaccharide impregnated with wax.
It is
the tectocuticle of Richards (1953);
it is a multilayer of which the inner most layer is
the monomolecular lipid layer of Locke (1966) and Gluud (1968),
the wax layer of Wigglesworth (1975).
Function of the epicuticular ornamentation
Epicuticular surface ornamentation of Tomocerus? sp.
Ghiradella, H.T. 2000
 |
Respiration under water.
The plastron respiration hypothesis of Imms (1906) or Noble-Nesbitt (1963).
When the littoral Anurida maritima and Anuridella marina
are submerged in water, a layer of air is retained in the
troughs between the microtubercles.
The very thin firmly held layer of air is termed a 'plastron'.
It is held in position by surface tension
forces and its volume remains constant (Wigglesworth, 1965:343)
as long as the gas exchange between the submerged animal and the plastron
is in balance with the gas exchange between the plastron and the
surrounding water;
the animal can continue to respire when submerged,
if the water is well aerated.
Collembola are terrestrial animals that do not normally enter water.
Many species are soil inhabiting.
In the soil they may be subjected by flooding due to rain.
Specimens survive flooding through an epicuticular plastron.
The plastron is a respiratory structure where the epicuticle is covered by
protuberances that retain a constant volume of air covering the cuticle
when the animal is submerged into water.
This thin sheet of air works like a gill:
oxigen depletion in the plastron air leads to the diffusion of more oxygen
from the surrounding water into the plastron.
The rigid epicuticular structures ensure that the air volume remains constant
and that the gas is not dissolved in the surrounding water.
Sminthurides aquaticus may 'slip' into the water and stay completely
submerged for about 4 days (Falkenhan, 1932 cited from Thibaud, 1970:181).
Cave Collembola may traverse over the bottom of water pools
(Franciscolo, 1951 cited from Thibaud, 1970:181).
Adult Arrhopalites may live 17 days under water
(Delamare Deboutteville 1952, cited from Thibaud, 1970:181).
When Ceratophysella armata exits from the water and returns back into
the water, its body is covered with a thin layer of air
(Pritt, 1951 cited from Thibaud, 1970:181).
Onychiurus, Tomocerus, and Orchesella breath under water
through the thin layer of air that surrounds them, that functions like a
"physical lung": the oxygen of the layer of air that is consumed due to the
respiration of the animal is renewed by the oxygen of the water
(Ruppel, 1953 cited from Thibaud, 1970:181).
Submerged specimens of Typhlogastrura balzuci postpone moulting for
36 days; those that attempt to moult do not survive it
(Thibaud, 1970:181-182).
Possibly, Hypogastruridae may complete their life cycle sub-aquatic
(Thibaud, 1970:182).
Cuticular plastron structures are also found in whip spiders (Amblypygi);
e.g. in the under stones on beaches of Florida occuring
Phrynus marginemaculatus,
surrounding the book lung stigmata
(Weygoldt, 2000:61).
The ability to stay alive under water for several hours may be an adaptation
to living close to water.
Epicuticular surface ornamentation
Sepsenwol, S. © 2004
 |
A comprehensive nomenclature concerning the epicuticular ornamentation
is suggested (
modified after
Massoud & Barra, 1980:252-2591;
and compiled from
Dallai & Malatesta, 1973:1367;
Eisenbeis & Wichard, 1985:204,2184;
Ghiradella & Radigan, 1974:3022;
Hale & Smith, 19665;
Hopkin, 1997:51-553;
Massoud, 19696;
Nickerl & al., 2012:4,59;
Paulus, 1971:38,398
):
- granules (grains1), minor tubercles3,5,
microtubercles3,
bosses2,
Mikrotuberkel4,
Grana8,
tubercoli minori7,
elementary mushroom-shaped elevations of the epicuticle
having a hollow columnar body that is topped with a flattened head
- primary granules9 (grains primaires1,6,
granules primaires6),
nanoscopic granules9:
primary granules having a triangular head (such as in Tomocerus minor cf. Massoud & Barra, 1980:255(Fig.2A)),
a square head (such as in Folsomides angularis cf. Massoud & Barra, 1980:253(Fig.1A)),
(quadrangles9),
or a rectangular head (such as in Anurida maritima cf. Hopkin, 1997:55(Fig.4.9a,b))
- secondary granules sensu Massoud, 1969 (grains secondaires6):
primary granules can merge together to form secondary granules
having a polygonal head;
note that primary square-headed granules can reduce to secondary
triangular-headed granules
- secondary granules sensu Nickerl & al, 2012:4,
microscopic papillose granules9:
the seconday granules form an additional hierarchic layer;
note that in this definition the secondary granules are not
epicuticular constructs but exocuticular/epidermal constructs.
Secondary granules are lacking in Entomobryomorpha (Nickerl & al, 2012:5).
- pillars3 (piliers1), raised triangular studs2:
a pillar is the columnar body of a granule;
the pillar is hollow and this lumen is connected with the underlying
transcuticular pore canal; the pore canal contains 3 to 10 wax filaments
that fuse together when penetrating the epicuticular pillar lumen
-
ridges3,9
(ponts1,
Brücken8),
boundary walls2:
linear connections between the pillars of the granules;
the ridges can be simple (with a demi-circular cross-section)
such as in Folsomia candida cf. Massoud & Barra, 1980:253(Fig.1B),
or double (with a saddle-shaped cross-section: demi-circular
with a central depression) such as in Orchesella ariegica cf. Massoud & Barra, 1980:255(Fig.2C));
the ridges are hollow and bear one or
two (see Note) longitudinal canals;
the ridges can be absent, such as in Coenaletes vangoethemi cf. Jacquemart, 1980:70(Planche II.5),
note that in this particular case raised polygonal hollow secondary
granules are formed by fusion of primary granules of adjacent crowns
-
Fig.5. Cuticular surface structure of Coenaletes vangoethemi
Jacquemart, S. 1980
 |
crowns (couronnes1,
Tuberkelringen4,
Wabenmuster8),
comb-like patterns of primary granules9:
a set of ridges forming a closed ring of interconnected pillars;
the basal tangential section of the crown is in principle circular,
the apical tangential section is more square or hexagonal;
the canals in the ridges are interconnected at the pillars to form
an epicuticular network of wax filaments
- alveoles (alvéoles1), troughs3:
the open epicuticular space surrounded by a crown
- arrangement3 (agencement1), comb structure9:
the topological pattern formed by the relative position of the granules.
Two types of arrangement can be distinguished:
- 1. primary arrangement: the arrangement of primary granules.
The regular crown is characterised by a constant number of
equidistant primary granules with identically shaped heads.
- 2. secondary arrangement: the arrangement of secondary granules.
The irregular crown is characterised by a random number of
random distant secondary granules with random polygonal heads.
Two types of primary arrangement can be distinguished:
* 1. hexagonal arrangement (hexagon9):
the regular crown is characterised by six equidistant
primary granules with triangular heads.
* 2. subrectangular arrangement (rhombic9):
the regular crown is characterised by four subequidistant
primary granules with subrectangular heads.
Rhombic patterns are typical in hemiedaphic Isotomidae (Nickerl & al, 2012:5).
The primary arrangement of granules is found at topological relatively regular
epicuticular fields, while the secondary arrangement is found
at locations where the epicuticula has to accomodate irregular
topological transitions.
Transmission Electron Micrographs (TEM's) of cross-sections of the
epicuticular ornamentation are not always that easy to interprete.
Depending on the angle relative to the cuticular surface the section was made,
the image may look quite different and it is not always obvious to reconstruct
from the image the three dimensional structure of the ornamentation.
To be able to interprete the images correctly,
it is required first to learn to recognise from the image
at what angle the section was made.
Sections can be classified according to several criteria
related to the plane the section was made:
- the angle relative to the cuticular surface:
- tangential sections,
- perpendicular sections,
- oblique sections.
- the angle relative to the epicuticular tesselation:
- crown median section, through the centre of a crown:
- perpendicular to opposite ridges of a crown,
- oblique to the ridges of a crown,
- through opposite granules of a crown.
- crown saggital section:
- perpendicular to the ridges of a crown,
- oblique to the ridges of a crown,
- through opposite granules of a crown,
- longitudinal through a ridge of a crown.
Fig.c1. Section of the epicuticula of Pseudosinella subduodecima
Barra, J.-A. © 1973 (unpublished)
 |
An example: Fig. c1 is a section of the epicuticular ornamentation
of a specimen of Pseudosinella subduodecima (Barra, 1973),
one day after moulting.
In the left part of the image, the section is
at a surface oblique tangential angle.
The 'air-bubble'-like objects are the oblique tangential projections
of the sectioned troughs of the crowns.
Note that these 'air-bubble' like artefacts have misled Paulus (1971)
in his interpretation of the three dimensional epicuticular structure.
In the centre of the image, the section becomes more surface oblique
perpendicular, indicating a slightly downwards curved local topography
of the cuticular surface
(note also the flattened cuticular horizon).
And at the right it is again more surface oblique tangential.
Given the relative wide ridges (in left and right part of image),
the section is crown saggital oriented.
Given the almost perfect symmetry of the tesselation,
the section is perpendicular to opposite ridges of a crown.
The asymmetry at the right is an indication of an anomaly in the
hexagonal tesselation.
Applying this interpretation to the section results in a projection
of the ornamental tesselation as represented in the schematic
diagram of Fig. 2c.
Fig.c2. Schematic projection of the ornamental tesselation deduced from the section of the epicuticula of Pseudosinella subduodecima
 |
Three dimensional Model of the epicuticular Ultrastructure
Fig.p. Three dimensional reconstruction
of
the exocuticula and epicuticula
Paulus, 1971.
 |
Paulus (1971:37-44) proposed a three dimensional model (fig. p) of the
epicuticular structure based on an interpretation of TEM images
of a tangential section through the cornea of Neanura sp.
and a cross-section through the cuticula of Entomobrya muscorum.
Paulus, apparently not aware that the cuticular cross-section was quite
oblique, misinterpreted the TEM image
and concluded that the epicuticula rests on short pillars.
See chapter "Interpreting cross-sections
of the epicuticular ornamentation" for a more elaborate discussion.
Paulus' model does support the plastron respiration hypothesis of
Imms (1906) or Noble-Nesbitt (1963).
Tuberculate Cuticula
Fig.8. Tuberculate cuticula
of Sminthurides sp.
Palacios-Vargas, J. © 2000.
 |
Fig.7. Tuberculate dentes
of Sminthurides sp.
Palacios-Vargas, J. © 2000.
 |
The hexagonal primary granular ultrastructure of the surface of the
epicuticle is a primary characteristic of the cuticula of all Collembola.
While the primary granules (minor tubercles or 'tubercoli minori' of Dallai, 1973)
are epicuticular structures,
the tubercles (major tubercles or 'tubercoli maggiori' of Dallai, 1973)
are exocuticular structures.
In the latero-ventral view of the mid part of the dens of
Sminturides sp. (Fig.7 after Palacios-Vargas, 2000:103(Foto 4))
each tubercle is covered by the epicuticula with its primary
granular ultrastructure.
Note also the lateral exocuticular folds.
Due to the curvature of the tubercular surface, the regular hexagonal
primary granular pattern is strongly deformed.
The largest deformation occurs at the apex of the tubercle.
Each tubercle is covered by a helicoidal texture of hexagons.
The helicoidal hexagon winding can be explained by a mechanism
purely based on geometrical considerations.
The most important geometrical constraint in this model is:
the hexagon formation process can be
viewed as analogous to winding strings around a cone in such
a way that its surface is fully covered. Therefore,
the number of strings being wound simultaneously per cone circumference
must be dependant on the angle they make with
a plane perpendicular to the cone axis.
A terminal pentagon closes the helicoidal winding at
the apex of the tubercle (Fig.7).
The tuberculation (Fig.8) can be seen as a secondary characteristic
of the cuticula.
Tuberculation originates from exocuticular folding.
Periodically spaced repeated linear folding results in crenulation.
Tuberculation can be described as the result of two orthogonal crenulations.
Tuberculation of the cuticula increases the surface of the cuticula
dramatically.
Assuming that tuberculation is a derived character,
the phylogenetic position of the Poduromorpha can be questioned.
Tubercles of Hypogastrura tullbergi
Fig.9a. SEM of tubercle
of Hypogastrura tullbergi
After Cassagnau, 1977.
 |
The cuticula of Hypogastrura shows one of the most basic primary tubercles
of all Collembola.
While the relative long tubiform tubercles of Sminthurides
are characterised by
an epicuticular helicoidal winding of hexagonal crowns,
the relative stout tubercles of Hypogastrura tullbergi with a more
tetrahedral architecture have three epicuticular hexagonal crowns
that are joined at the top vertex of the tetrahedron
(the apex of the tubercle)
(see Fig.9a after Cassagnau, 1977; planche II, fig. a; x 11,000:
a tubercle of the tergite of the fourth abdominal segment).
The hexagonal primary arrangement in Hypogastrura tullbergi
is constituted of triangular headed primary granules.
The tubercles are more elevated than the triangular primary granules,
and have a superimposed hexagonal arrangement of triangular primary granules.
The triangular head at the apex of the tubercle is more raised
than any other primary granule and its size is much larger.
Fig.10a. 2D scheme of tubercle
of Hypogastrura tullbergi
Janssens, F., © 2001.
 |
Based on the SEM of a tubercle (fig.9a)
a simplified two dimensional scheme of the epicuticular arrangement of the
primary granules on the tubercle
can be represented as a kind of exploded, flat view
(fig.10a: top view that can be mapped to the SEM of fig.9a).
Three regular hexagonal crowns are joined at the center.
This center is the apex of the tubercle.
|
The three dimensional epicuticular arrangement of the primary granules
is modelled as a ball and stick wire-frame.
The sticks represent the ridges interconnecting the primary granules.
The balls represent the 3D position of the primary granules.
The heads of the primary granules are modeled as intersected tori
that are placed at the ends of the sticks.
In this way, by putting the sticks together in the proper
geometrical configurations, the polygonal heads of the primary granules
are modelled implicitly. Not only the shape but also the size of the heads
are properly modelled: concave joined sticks result in smaller heads;
convex joined sticks result in larger heads.
The model allows us to formulate a relation between the size of the
primary granular head and the angle between the longitudinal axes of
the ridges connected to the granule:
s = r*(1+2*sin(30+(alpha/2)))
- alpha is the angle between the longitudinal axes of the sticks;
- s is the size of the primary granular head measured from
the center of the head to the edge orthogonally.
This formula is based on the follwing simplified assumptions
on the dimensioning of the ridges:
- radius of the sticks, minor radius of the tori
and radius of the balls are all equal (r);
We can also derive the the type of cuticular surface curvature
from the variability of the size of the primary granular heads.
Given a regular primary arrangement of primary granules,
heads that are smaller than those of the regular primary arrangement
indicate a concave cuticular surface
while larger heads indicate a convex cuticular surface.
|
Fig.11b. 3D model of tubercle
of Hypogastrura tullbergi
lateral view
Janssens, F., © 2001.
 |
Fig.11a. 3D model of tubercle
of Hypogastrura tullbergi
top view
Janssens, F., © 2001.
 |
The tubercle is modelled as a flat topped tetrahedron.
At the top of the tetrahedron three hexagons of primary granules
intersect in such a way that a small platform is made that serves as
a carrier for the large triangular head of the tubercle
(fig.11a: top view that can be mapped to the SEM of fig.9a;
fig.11b: lateral view).
Note that this apical triangular head is that large due to the fact
that it is the combination of three convex tilted primary granules.
Note also that the three triangular heads at the corners of the platform
are the combination of three convex tilted primary granules.
Due to the fact that they are relatively less tilted,
they are smaller then the apical head,
but still larger than the regular heads.
The bases of the hexagons line-up with the hexagonal primary arrangement
of the triangular primary granules of the non-tubercular epicuticula.
Tubercles of Podura aquatica
Fig.9. SEM of tubercle
of Podura aquatica
After Dallai & Malatesta, 1973.
 |
The tubercles of Podura aquatica
are quite larger than those of Hypogastrura:
three epicuticular hexagonal crowns are joined
at the top vertex of the tetrahedral tubercle
(the apex of the tubercle).
The hexagons are extended with two pentagonal crowns at the
base of the faces of the tetrahedron
(see Fig.9 after Dallai & Malatesta, 1973; x 10,000).
The tetragonal primary arrangement in Podura aquatica
is constituted of square headed
primary granules (Dallai & Malatesta, 1973:136).
The tubercles
(major tubercles cf. definition of Hale & Smith, 1966, or
granules secondaires cf. Massoud, 1969, or
tubercoli maggiori cf. Dallai & Malatesta, 1973)
are more elevated than the tetragonal primary granules,
and have a superimposed hexagonal/pentagonal arrangement
of triangular primary granules
(Dallai & Malatesta, 1973:136).
The triangular head at the apex of the tubercle is more raised
than any other primary granule
and its size is up to 5 times larger, up to 400 nm long
(Dallai & Malatesta, 1973:136).
Effectively, the cuticula of Podura and in particular
the formation of the tubercles is realised
according to a scheme that is analog to that observed
in Hypogastruridae
(Dallai & Malatesta, 1973:137).
Fig.10. 2D scheme of tubercle
of Podura aquatica
Janssens, F., © 2001.
 |
Based on the SEM of a tubercle (fig.9)
a simplified two dimensional scheme of the epicuticular arrangement of the
primary granules on the tubercle
can be represented as a kind of exploded, flat view
(fig.10: top view that can be mapped to the SEM of fig.9).
Three regular hexagonal crowns are joined at the center.
This center is the apex of the tubercle.
The hexagons are surrounded by irregular pentagons.
In this simplified scheme,
all pentagons are identical and
are derived from the regular hexagons.
The bases of the pentagons line-up with the tetragonal primary arrangement
of the primary granules of the epicuticula.
The intrinsic triangular nature of the tubercle is clearly demonstrated.
Fig.11b. 3D model of tubercle
of Podura aquatica
lateral view
Janssens, F., © 2001.
 |
Fig.11a. 3D model of tubercle
of Podura aquatica
top view
Janssens, F., © 2001.
 |
The three dimensional epicuticular arrangement of the primary granules on
the tubercle are modelled as
a ball and stick wire-frame
(fig.11a: top view that can be mapped to the SEM of fig.9;
fig.11b: lateral view).
At the top of the tetrahedron, three regular hexagons of primary granules
intersect in such a way that a small platform is made that serves as
a carrier for the large triangular head of the tubercle.
Note that this apical triangular head is that large due to the fact
that it is the combination of three convex tilted primary granules.
Note also that the three triangular heads at the corners of the platform
are the combination of three convex tilted primary granules.
Due to the fact that they are relatively less tilted,
they are smaller then the apical head,
but still larger than the regular heads.
This architecture is similar to that of Hypogastrura.
However, the podural tubercle is more than twice as high
as the hypogastrural tubercle.
It is also more steep.
As a result, each hexagon is at its basis extented into the face of the
tetrahedron with two pentagons that form
the basis of each face of the tetrahedral tubercle.
At the flanks of the tetrahedron, the faces are joined through
strongly deformed pentagons.
The base pentagons line-up with the tetragonal primary arrangement
of the tetragonal primary granules of the non-tubercular epicuticula.
Tubercles of Tetrodontophora bielanensis
Cf. Dallai (1973):
steep conic shape of tubercle: Tav. II fig 1
tetragonal arrangement of primary granules: Tav. II fig 2 and 3
primary granules with tetragonal head: Tav. II fig 2 and 3
resembles tubercles of Podura aquatica
but more elevated
base of tubercle = pentagonal
less elevated tubercles on ventral side of furca: Tav. VI fig 2
with tetragonal base
and 4 uptilted pentagons joined at the apex of the tubercle
apical granular head = tetragonal and much larger than
the other tetragonal heads of the granules of the primary arrangement
To be completed.
To be completed.
Acknowlegdements
We would like to thank Dr Nico Büsscher for preparing the in paraplast
sectionned specimens.
References
-
-
-
-
-
- Edney, E.B. 1977.
Water balance in land Arthropods., in Zoophysiology and Ecology, Vol.9, p.42-57.
-
-
-
-
-
-
-
- Snodgrass, R.E. 1935.
The Principles of Insect Morphology,
Chapter III,
The Body Wall and its derivatives.
(Ed.) Schouest, L. Last updated Dec. 4, 1996.
-
-
 |
Fig.x. Animation to show that the
hexagonal pattern of epicuticular ridges improves the tangential rigidity
of the cuticle.























